Hydrogen is a fascinating element with many interesting properties and applications. Here are some intriguing facts about hydrogen:
- Lightest Element:
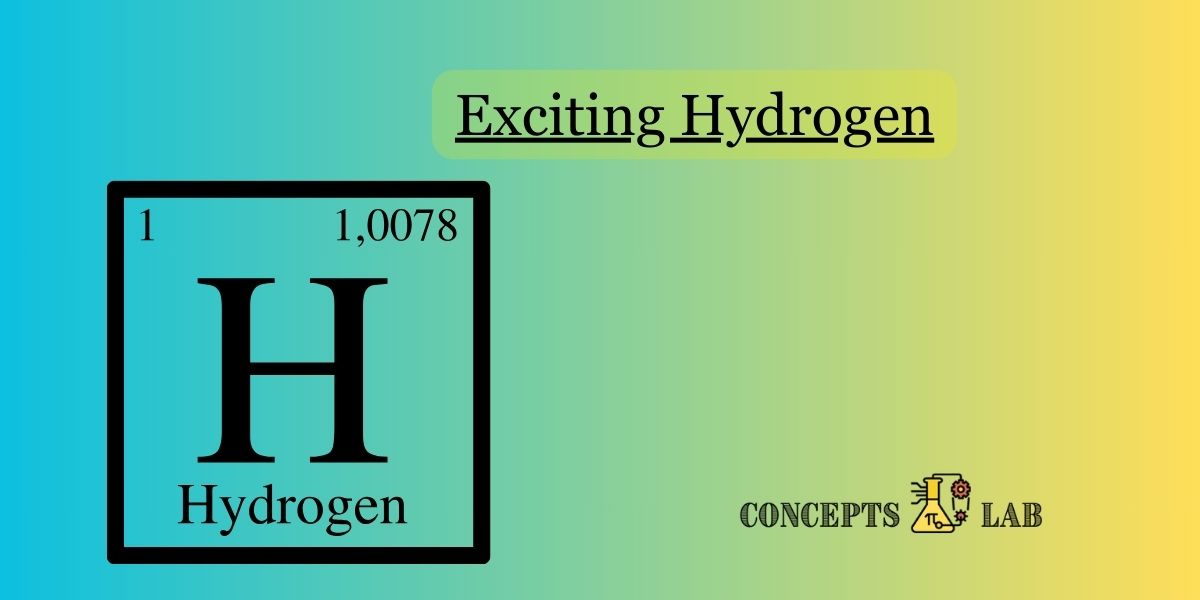
- Hydrogen is the lightest and simplest element in the periodic table. Its atomic number is 1, and it consists of only one proton and one electron.
- Abundance in the Universe:
- Hydrogen is the most abundant element in the universe, constituting about 75% of its elemental mass.
- Colorless, Odorless, and Tasteless:
- In its pure form, hydrogen is a colorless, odorless, and tasteless gas. It is invisible to the naked eye.
- Clean Energy Source:
- Hydrogen is often considered a clean energy carrier. When it reacts with oxygen in a fuel cell, it produces water and releases energy, making it a potential alternative fuel for vehicles and power generation.
- First Element in the Periodic Table:
- Hydrogen occupies the first position in the periodic table. Its simplicity and unique properties make it an essential element for understanding the structure of matter.
- Diatomic Molecule:
- In its natural state, hydrogen exists as a diatomic molecule (H₂), meaning it consists of two hydrogen atoms bonded together.
- Liquid Hydrogen:
- Hydrogen becomes a liquid at extremely low temperatures, close to absolute zero (-273.15°C or -459.67°F). Liquid hydrogen is used as rocket fuel.
- Balloon Lifting Power:
- Hydrogen has been historically used as a lifting gas in balloons due to its lightness. However, its flammability led to safety concerns, and helium is now the preferred gas for balloons.
- Role in the Sun:
- The sun primarily consists of hydrogen undergoing nuclear fusion to form helium. This process releases an enormous amount of energy, providing light and heat to our solar system.
- Hydrogen Bonds:
- Hydrogen is known for forming hydrogen bonds with other atoms, particularly with oxygen and nitrogen. These bonds play a crucial role in the structure of biological molecules like DNA and proteins.
- Hydrogen in Water:
- The name “hydrogen” itself comes from the Greek words “hydro” (water) and “genes” (forming). This reflects its role in the formation of water (H₂O) when it combines with oxygen.
These facts highlight the versatility and significance of hydrogen in various scientific, industrial, and environmental contexts.

No responses yet